Getting the most out of your sales and marketing technologies—so you can sell more in the life sciences – Part 1
By David Chapin
SUMMARY
VOLUME 9
, NUMER 2
Is your life science CRM system working optimally? Are you tracking your new business opportunities effectively? Do you have the necessary visibility into your sales pipeline? Is everyone on your life science sales team using your system in a consistent manner? If the answer to any of these questions is “no,” then you’ve got a protocol problem—or is it a personnel problem? Or maybe it’s a technology problem? Let’s face it: CRM systems are complex beasts, with lots of moving parts, including software, protocols and personnel. Many life science organizations fail to use them effectively. In this first part of a series, I examine how to assess where the challenges arise in the adoption of proper CRM processes inside life science organizations, and what you can do about them. In future issues we’ll dive deep and develop a plan for getting the most out of your CRM system. The goal of all this: to tune your tools, so you can be more effective in your marketing and new-business development efforts.
The promise of new sales and marketing technologies in the life sciences
I want to talk about one of the big challenges faced by many life science organizations: getting the most out of the innovative marketing technologies that are frequently purchased but seldom utilized to their fullest extent.
The promise of new marketing technologies—such as Customer Relationship Management software (CRM) and Marketing Automation technology (MAT)—is incredibly seductive. Just imagine: all your business development employees enter their leads into your database in the same way, and they all follow-up using clearly defined protocols. Imagine the visibility this would give you into the state of your sales pipeline. And just imagine that all prospects are assigned to a clearly defined Marketing Automation workflow, each of which is optimized to nurture that prospect according to their stage in the buying cycle and their particular interest. Imagine the results you could achieve.
This vision of the future is seductive, isn’t it? This is the enticing vision that the CRM salespeople promise. It’s a savory mix of many desirable benefits—Efficiency, Consistency, Responsiveness, Clarity and Visibility, among others. And if you achieve these benefits, then by implication you’ll also achieve outstanding results, such as greater Awareness and improved Closing Ratios.
This promise is not empty. You really can achieve the promised benefits. But doing so isn’t simple. Unfortunately, this means that many life science companies don’t achieve the results they were promised in those glossy sales pitches.
For organizations in the life sciences that have purchased a complex software solution, there are two main sticking points: initial implementation and ongoing adoption. Immediately following purchase, implementation is the short-term challenge; most organizations can get through the implementation phase in a matter of months, using a small team. But proper adoption is a longer-term challenge. Proper adoption requires that you align employees from multiple functions, and include your entire sales and marketing teams, if you’re going to succeed. Because adoption is the place many organizations stumble, I’ll leave initial implementation for another issue. Today I’m focusing on adoption, and how you can achieve the best possible results.
I’m making some assumptions here. I’m assuming that you’ve already bought a CRM, and you’ve gotten through the preliminary implementation phases. The challenge that’s facing you now is one of proper adoption—that is, making sure your life science organization is getting the most out of your investment in your CRM.
As an aside, it can often be the purchase of additional technology—Marketing Automation technology (MAT)—that forces the realization that your current CRM protocols are flawed. This is ironic, because you can’t make the most out of your MAT without solid CRM processes. If you find yourself in this situation, you’re faced with tuning multiple systems at once. But let’s not get ahead of ourselves. Your CRM is often the repository of data that drives the MAT: garbage in, garbage out. So, for this issue, I’m going to focus on CRM adoption in the life sciences.
The shifting relationship between sales and marketing in the life sciences
CRM and MAT systems can be confusing. Why? Their use tends to redefine the lines between the sales and marketing functions, so it’s worth spending just a minute to clarify the differences between sales and marketing.
In those sectors of the life sciences where price tags are high and a “consultative sale” is the norm, marketing’s role is to build awareness, establish contact and build trust with prospects—and then to pass along to the sales team those life science prospects who are deemed ready for further qualification. In other words, one of marketing’s prime roles is to generate leads. Leads not yet ready to buy continue to be nurtured by marketing.
There are many ways to generate leads in these life science sectors: buying a list, collecting business cards at a trade show, collecting leads from the conversion forms on a website, etc., etc. Once the leads are collected, marketing rates these leads, determining what type of follow-up is required. These decisions are based on multiple factors, some precise and some not. For example, one prospect may fill out a form on your website and include some information that indicates they’re already deeply involved in the buying process. In this case, immediate follow-up is required, so the lead would be quickly passed to sales. In contrast, a list of 5000 recent life science trade show attendees can’t all be qualified on the phone, so the follow-up might consist of emailing them provocative thought leadership once a month (once they’ve given their permission, of course).
The leads passed on to sales are known as marketing qualified leads (MQL), because the leads have been “qualified” by the marketing function.
That’s marketing. And sales? The role of sales is simple: to close business.
To do this, the Sales function must qualify those leads deemed ready for qualification. Depending on the status of the prospect, they then help transition the lead from one stage in the buying cycle to the next—from Stage 2 (Contemplation) all the way to Stage 4 (Action), when the opportunity is closed (meaning: won, lost or returned to an earlier stage).
You may be wondering, “Why is a firm known for marketing in the life sciences taking it upon themselves to tell me about CRM adoption? Isn’t proper CRM adoption in the life science an issue for the sales function, not marketing?”
Good question. There are two reasons for this, one structural and one practical. Over the last decade or two, the marketing and sales functions have become more tightly linked. As evidence, consider that in many life science companies these two functions now report to same person in the C-suite. That’s not true in the typical consumer product goods companies, by the way. That’s the structural reason; here’s the practical one: given the tighter link between sales and marketing, it’s difficult to implement effective marketing automation practices without good data in the CRM. And it’s difficult to manage your sales team effectively without good data in the CRM. Do you sense a pattern here?
The importance of consistency in CRM processes and protocols in the life sciences
The leads that are passed from the marketing function to the sales function live in the CRM. It is crucial that these leads—the high-value, future lifeblood of your life science business—be maintained properly. But—at the risk of generalizing—the people who gravitate to sales and marketing are not naturally detail-oriented. Exceptions do exist, but to oversimplify, marketing people tend towards creativity, while sales people are relationship-driven.
What does this mean? In many life science organizations, it means that the CRM system as a whole is a mess. Each salesperson has their own system and rules-of-thumb, which aren’t consistent with any of the other processes used by other sales people. As a result, visibility into the state of the organization’s pipeline is cloudy, and visibility into the effectiveness of any one salesperson is poor. I won’t ask you to raise your hand, but does this sound like your organization?
To determine the seriousness of your situation, ask yourself these questions:
- Does each and every salesperson agree on the terminology and systems used to enter a prospect into the database? To find out, pick some recent entries by different salespeople and audit them. You may have to talk to individual salespeople to clarify the nature of the lead. Are they classifying entries correctly? Do they have a set of principles to guide them in their use of the CRM?
- Can you easily determine where each prospect is in the buying cycle? Another way to ask the same question: can you easily view your entire “sales funnel?” Can you easily determine how many people there are at each stage of the buying process, how long they’ve been at each stage, and what actions have been taken (and by whom) to nurture them? To ascertain this, run two pipeline reports, a month apart. Then compare these against all recent entries into the database. If you have recent entries that don’t show up in the pipeline report, then what’s going on? Are people entering information correctly?
If the answers you turn up give you pause, it’s time to take stock—if you don’t fix this situation now, it will only get worse. So how should you proceed?
Taking stock of your CRM processes, protocols and personnel in the life sciences
In addition to the two questions listed above, begin by establishing whether your team’s efforts are aligned or not. Let’s start by asking questions around the following crucial topics:
- capturing the right information
- doing so consistently
- clarifying the process
- sharing the understanding of the process
- keeping your information clean
- reporting
Ask yourself (or your team) these questions:
- On a scale of 1-7, how completely do we capture the information we need in order to manage the sales process effectively?
- 1: We’re lucky if we record any of the information we really need; consequently, our database isn’t very useful.
- 7: We record everything we truly need, and nothing we don’t use.
- On a scale of 1-7, how clearly defined is the process for qualifying new life science leads?
- 1: Not clearly defined; for example, we don’t use common terminology.
- 7: Clearly defined; for example, the terminology we use to label things is clear.
- On a scale of 1-7, how well is the process for qualifying new leads understood by our entire life science business development team?
- 1: There is no shared understanding about how leads are qualified; everyone has their own system.
- 7: We all follow the same system, and we review the system periodically to ensure that we’re all compliant.
- On a scale of 1-7, how clean is our database (lack of duplicate records, all information is accurate)?
- 1: Our database is a mess!
- 7: Our database is clean, and we do regular maintenance to keep it that way.
- On which of the following topics do we run regular reports out of our CRM?
- Sales cycle report, showing the length of our sales cycle.
- Pipeline report, showing how our leads are progressing through the buying cycle, and what opportunities are pending.
- Sales forecast report, projecting future revenue.
- Sales conversion reports, telling us what percentage of leads convert into a closed deal.
- Pipeline Next Steps, showing the next steps for each open opportunity.
(Note that this whitepaper addresses your life science CRM, so I’m not including in the reports listed above the valuable information such as Campaign effectiveness rate, or Conversion rate, or a whole host of others. These reports originate with your marketing automation system, not your CRM.)
Please note that these are not the only possible diagnostic questions you could ask, but they are a good place to begin because they focus on the most-important issues. In addition, you should pay attention to criticisms you’ve received about your CRM. Your life science team members will frequently complain about how difficult it is to use your CRM. I’ve found that once you dig into the issue, the real problem usually isn’t “ease of use,” but something else entirely, like insufficient training or inadequately defined protocols. These “ease of use” complaints are a good hint that something else is going on.
To score your responses above, add all the scores for answers for questions 1-4, and add an additional point for each answer selected in question 5. Your total will be between 4 and 33. If your score is lower than 25, then you need to consider remedial action. If your score is below 18, then you’re past the point of “considering” a solution; you need to take action.
Note: You may choose to hire support to help you with this issue. But for most life science organizations, help was required during the implementation phase. Now, in the ongoing struggle to maintain the database, the problems may not be fixable by an outside firm, because the problems lie with your team’s day-to-day operations.
Adoption of proper CRM protocols in the life sciences
Adoption is a multi-faceted challenge. Some facets are related to procedure and protocol, while others are related to personnel. These facets interact; without good procedures, your personnel won’t be able to adopt the proper behaviors successfully. And without willing personnel, your procedures won’t do you a bit of good.
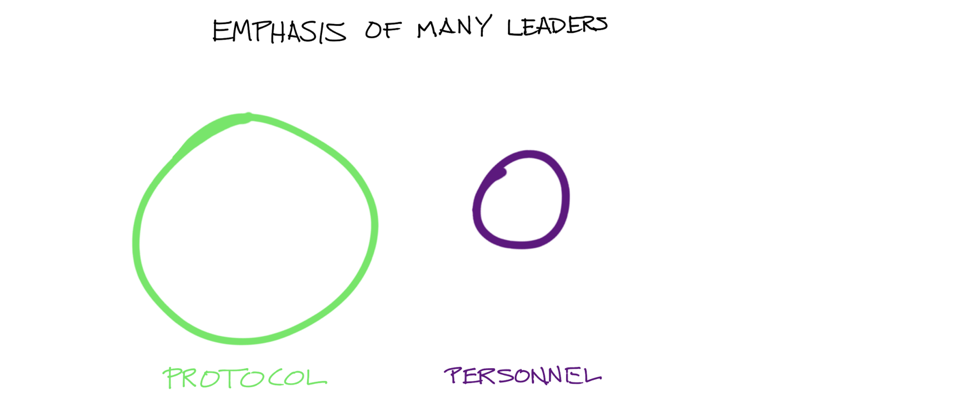
Unfortunately, many life science organizations put too much emphasis on protocol, and not enough on personnel:
Title: The emphasis of many leaders focused on CRM adoption is often skewed. For example: “Our protocols aren’t being followed so let’s rewrite them. I want to build in incentives to encourage proper compliance.”
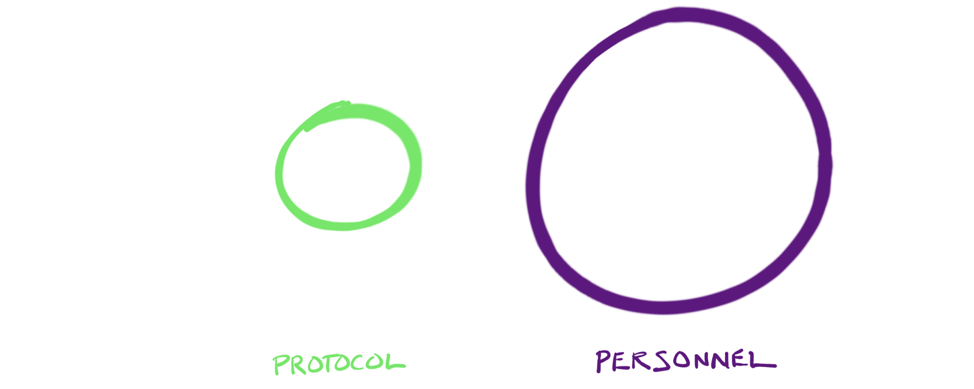
But failures of adoption are more often connected to issues under the control of personnel than they are to issues of process, protocol and procedure.
Title: The challenges of adoption most often arise from issues relating to personnel, such as considering the part that each person plays in the adoption process, engaging their emotions, and making sure that they understand the importance of the part they play.
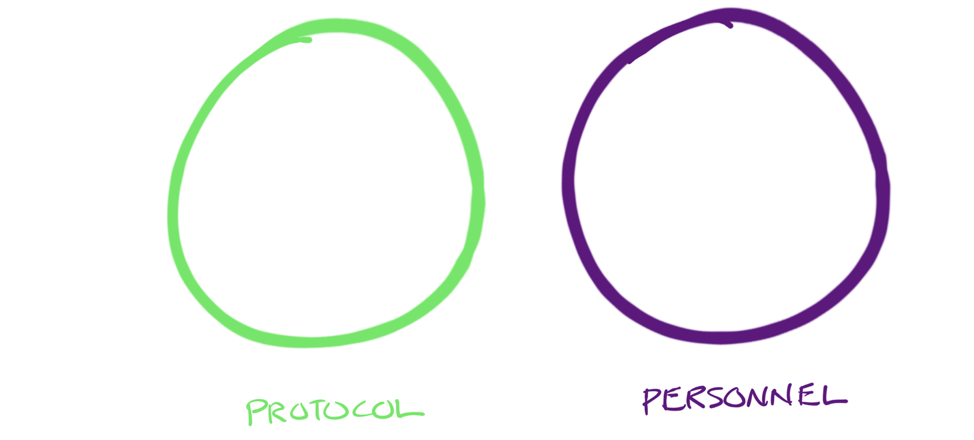
Creating the conditions for proper adoption requires an understanding of how to create meaningful protocols—not so detailed that they impede motivation, and not so loose that they’re worthless. And we must create the motivation to follow the protocols. Which means we need the proper balance, like this:
Title: An ideal balance focuses on both protocols and the personnel that must create, implement and monitor the protocols.
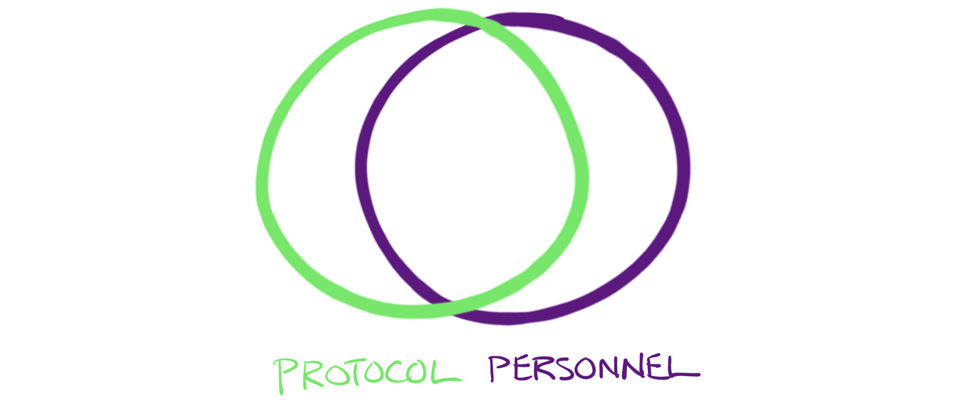
And as we’ll see, these issues are intimately related, like this:
Title: To ensure proper adoption, protocol and personnel must be properly aligned, each with the right amount of emphasis. We’ll explore this further in this and future issues of this whitepaper.
A step-by-step process for improving your CRM adoption
Here are the key steps you’ll need to follow to improve the adoption of the CRM in your life science organization. We’ll cover the initial step in this issue, and the remainder of this process in future issues.
- Audit
- Your current processes, protocols and personnel
- Dream (and plan)
- Identify what’s wrong, contrast this with an ideal future state, and plan the transition between the two
- Organize
- Create and document the desired process/protocol and your expectations
- Identify
- Identify key personnel. Align them through training.
- Implement
- Provide reinforcement. Measure and publish the appropriate data.
- Recognize success
- Reward and celebrate
- Iterate
Rinse and repeat.
Step 1: Audit the current processes in your life science organization
Many life science organizations fail to realize the extent of preparation necessary for proper adoption of a CRM, and so they find themselves having to go back and patch up the processes they failed to implement correctly in the first place. Rather than trying to address your problems as you come across them, I urge you to begin by performing a complete audit of your current processes. What’s working well? What’s not working at all? Clarify this and you’ll have a clear view of your starting position. Which will help you figure out how to get where you want to go. You’ll also be able to identify any components that depend upon others, so you can tackle the solutions in the proper order.
To begin your audit, collect any documentation related to your CRM protocols and procedures. What’s that you say; you don’t really have any documentation? Hmm, isn’t that part of the problem?
Conducting the proper audit in your life science organization: gathering input
If you have no documentation, then auditing your procedures will quickly devolve into a series of discussions as you wander from salesperson to salesperson asking about the details of their procedure. These discussions will further devolve into complaint session(s). This is what the Partners in Leadership authors Roger Conners, Tom Smith and Craig Hickman call “Below the line” behavior. These authors have collaborated on many books about creating an accountable organization, including The Oz Principle—Getting results through individual and organizational accountability, and How did that Happen? Holding people accountable for results the positive, principled way.
Behaviors that these authors consider “below the line” include:
- cover your tail
- wait and see
- confusion/just tell me what to do
- it’s not my job
- ignore/deny
- finger pointing
Behavior that is below the line may make participants feel good temporarily, because they don’t have to take responsibility for their own actions. But ultimately this behavior won’t help improve the situation. If you’re going to harness your life science team’s efforts to address the problem, you’ve got to do more than enable them to complain.
To avoid this, I suggest a two-prong approach. First, conduct some quick research with the individuals that are using your CRM. Ask them a couple of simple questions.
- How broken is the usage of our CRM system? (If you want, you can probe this using a Likert scale as in the survey above: On a scale of 1 (completely broken) to 7 (It’s not broken at all), how broken is the usage of our entire CRM system, from selection of the CRM tool, to the way we use it, to the training we’re given, to the information we get from it?)
- If we could fix it, what would the three biggest benefits be to you personally?
You are looking for two key data points here. First, you want to understand if people agree that there is a problem. After all, if they don’t see a problem, they’ll have no motivation to fix it, ever.
If they agree there is a problem, then you’ve got what Partners in Leadership calls an accountability gap: “a gap in performance that directly affects the organization’s ability to achieve a key result.”
Second, you want to see if there is agreement among your team about what the benefits would be. If there is a common agreement on a benefit or two, you can use this as a common point of agreement and a motivational tool.
Conducting the proper audit in your life science organization: Identify your team’s frustrations
A significant part of aligning your team (and following protocol) has less to do with logic and more with engaging their emotions.
If you’ve completed the first phase of your audit, the next phase involves a group input session. Assemble your entire team, specifically, anyone who’s part of the current problem or should be part of the solution. Explain that you’re trying to optimize the process. Stress the two data points that you learned in the research you just completed: namely, that people agree there’s a problem, and they agree on some common benefits, if the situation could be fixed. It’s important to stress that the point of this exercise is to deliver these benefits, such as easier compliance, better visibility into data, etc.
In doing this, you will have accomplished a couple of important things. First, asking people for their opinions and then showing them that you listened carefully to their answers helps them understand that their participation matters. Second, emphasizing the common benefits helps people realize that they really are all “in this together.” The issue is framed as a common one, and there are group-wide benefits to finding a solution.
In the language of Partners in Leadership, we have just taken several steps that are important to building accountability:
- obtaining the perspective of others
- asking for and offering feedback
- being personally invested
Okay, you’ve gotten everyone together for an input session. How can you avoid having this session turn negative? By imposing structure on the thoughts that are shared and captured. Here is one way to do that: Divide the whiteboard into thirds with vertical lines. Or, set up three large pads on easels. Title the left-hand column (or pad) Issue; the middle column Result (Impact); and the right-hand column Potential solution(s).
Fill in the chart row by row. In other words, enter an issue, a result (or impact) and one or more potential solutions. In each row, all entries should all be related to one another.
Here is an example of a couple of topics that might appear:
| Issue/Challenge | Impact/Result | Potential Solution(s) |
| Compliance with data entry is poor; different team members entering data differently. | Visibility into our sales pipeline is poor.
We can’t trust the data that we do look at. It’s hard to examine or compare opportunities that have been entered by different salespeople, because there’s no consistency. |
1) Streamlined practices simplify data entry; common terminology ensures data can be trusted. Training to ensure consistency. Follow-up is offered to help people who are having trouble.
2) We hire a data entry clerk to enter all the data to ensure consistency. 3) We hire a data entry clerk to check all the entered data to ensure consistency. |
| System is hard to use
It’s unclear what is important to management; the messages we get about this change weekly. |
Shortcuts get taken so I can get back to selling | 1) An easier system is installed and we are all properly trained on its use.
2) Management makes its expectations clear |
It’s important that your team fills out the whiteboard row by row across the board or pads. Don’t fill it out by going down a column. If you do, you’ll get lots and lots of complaints (disguised as issues) in the left-hand column. This will take up most of the meeting. Then people will offer a few results for the middle column. By this time everyone will be tired (and cranky from complaining so vociferously) and very few potential solutions will be entered in the right-hand column.
You want your life science team to recognize the problem, understand its impact and—most important—focus on offering solutions. In fact, require that anyone that volunteers an issue for the left-hand column also must provide both a result/impact (middle column) and one or more solutions (on the right). Participants don’t just get to complain; they’ve got to offer a solution. No solution, no complaining.
The goal of this session is not to solve the problem. The goal of this session is to involve people and help them see the bigger issue. You want them to understand how they are contributing to the problem. Helping them gain the understanding that the problem really does belong to them is, as explained by Partners in Leadership, one of four crucial steps towards closing the accountability gap.
Summary of auditing your life science organization’s CRM challenges
You didn’t arrive at this situation overnight, so fixing it won’t be quick. But it is possible. It begins with auditing your current situation. In this issue I’ve discussed some tools you can use to audit your current situation. It begins by asking whether you and your life science team are capturing the right information, and storing it in a consistent manner. If you are, you’ll set yourself up for the benefits I discussed earlier: Efficiency, Consistency, Responsiveness, Clarity and Visibility.
In the next issue, we’ll cover in greater depth a detailed process to get the most out of your life science CRM. I’ll address how you can harness employees’ motivations to help you develop a system that works—and sticks.
The Marketing of Science is published by Forma Life Science Marketing approximately ten times per year. To subscribe to this free publication, email us at info@formalifesciencemarketing.com.
David Chapin is author of the book “The Marketing of Science: Making the Complex Compelling,” available now from Rockbench Press and on Amazon. He was named Best Consultant in the inaugural 2013 BDO Triangle Life Science Awards. David serves on the board of NCBio.
David has a Bachelor’s degree in Physics from Swarthmore College and a Master’s degree in Design from NC State University. He is the named inventor on more than forty patents in the US and abroad. His work has been recognized by AIGA, and featured in publications such as the Harvard Business Review, ID magazine, Print magazine, Design News magazine and Medical Marketing and Media. David has authored articles published by Life Science Leader, Impact, and PharmaExec magazines and MedAd News. He has taught at the Kenan-Flagler Business School at UNC-Chapel Hill and at the College of Design at NC State University. He has lectured and presented to numerous groups about various topics in marketing.
Forma Life Science Marketing is a leading marketing firm for life science, companies. Forma works with life science organizations to increase marketing effectiveness and drive revenue, differentiate organizations, focus their messages and align their employee teams. Forma distills and communicates complex messages into compelling communications; we make the complex compelling.
© 2024 Forma Life Science Marketing, Inc. All rights reserved. No part of this document may be reproduced or transmitted without obtaining written permission from Forma Life Science Marketing.